About Us
HHAO MEDI® has been specialising in the development and manufacturing of medical devices since 2009 in China. Our main focus is on MRI-compatible devices, and we offer a wide selection of non-magnetic, non-ferrous equipment. These products are engineered to meet stringent safety standards while addressing the unique requirements of various healthcare facilities. We are dedicated to maintaining a high-quality management system in accordance with DIN EN ISO 13485 and are proud to be a CE-certified organisation.
Actualising Our Mission:
For over 12 years, we have specialised in manufacturing and supplying high-quality, cost-effective MRI products and equipment to facilities across more than 60 countries. Our company maintains a representative presence in both Italy and the United Kingdom. Our products are registered through our business partner in the UK and are fully compliant with the Medical and Healthcare Products Regulatory Agency (MHRA) regulations. (https://aayanmedicalimaging.co.uk/mri-safe-medical-equipment-uk/)
Our team brings extensive expertise, with over 12 years of experience in the MRI industry. This background enables us to offer a diverse range of products designed to enhance efficiency and workflow for our customers. We adhere to comprehensive testing protocols and uphold the highest quality standards, ensuring reliable performance for MRI applications rated up to 7.0T and below.
Our Values
At HHAO MEDI®, we believe in working as a family. Those who work with us are not just “human capital” or small wheels turning inside a large machine — they are valued members of our team.
We stand together through both professional and personal challenges, supporting one another with trust, respect, and compassion. We aspire to act, think, and feel like a true family unit.
We are the HHAO MEDI® Family.
And for us, the customer is always KING!
Manufacturer : NANNING HHAO TECHNOLOGY CO.,LTD
NANNING CHINA
Branch Office : HHAO MEDI CO LIMITED
HONGKONG CHINA
UK Branch Office( EUROPE) : AAYAN MEDICAL IMAGING LIMITED
MALDEN,KT3 3ST UNITED KINGDOM
Latest CE with MDR 2017/745, MR compatibility report, Italy registration certificate in the year of 2021

EC Declaration of Conformity:


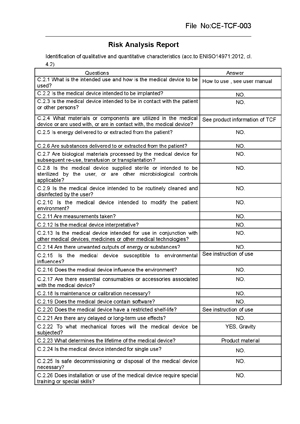
Risk Analysis Report Test report:

ISO: Free Sales Certificate:


Thailand CFS: VIETNAM CFS:


EU SFC

VEITNAM REGISTRATION CERTIFICATION:


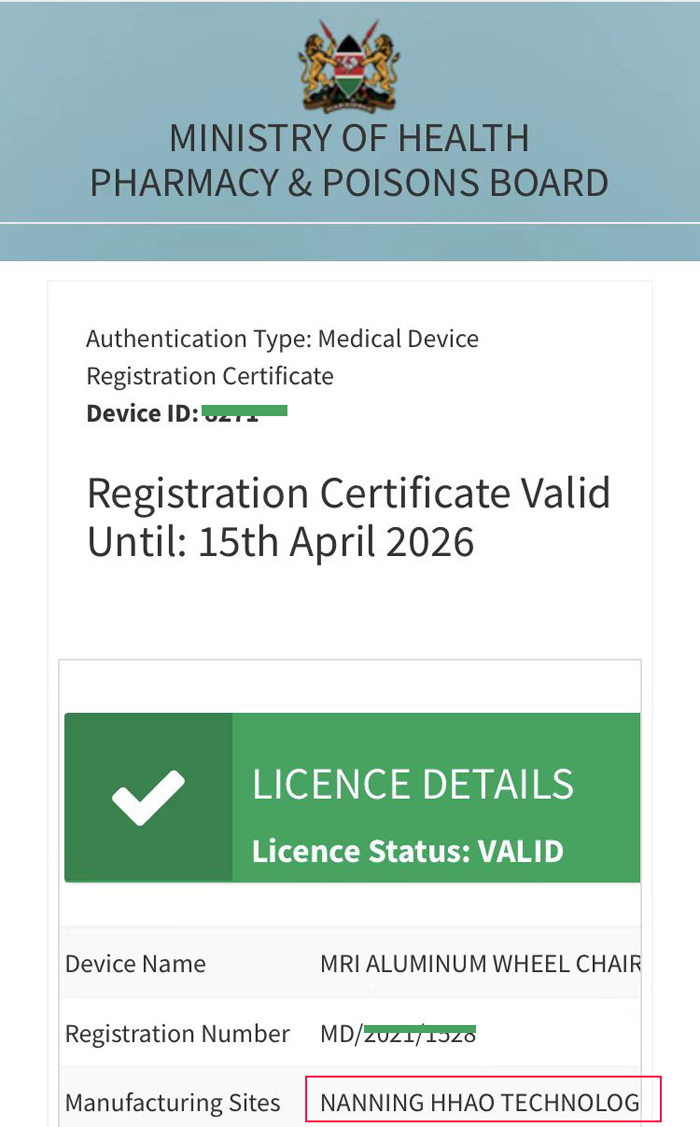
Australia TGA CERTIFICATION: Kenya PPB registration certificate:


 Sarah
Sarah Sarah
Sarah